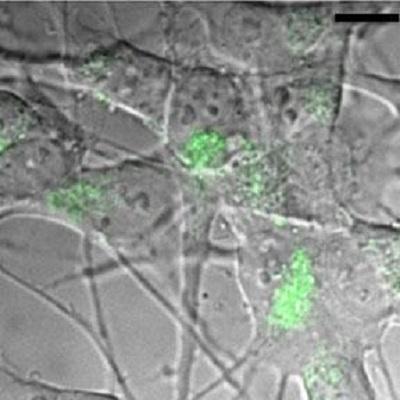
A powerful laser imaging technique developed by the group reveals how minute quantities of a protein associated with Alzheimer’s Disease trigger a process which may be crucial to its onset and spread.
In a recent paper just published in the Journal of Biological Chemistry we have been able to show that the protein Tau, a key protein at the focus of Alzheimer's disease (AD), propagates between cells in a manner similar to prion like proteins. The “prion-like hypothesis" of amyloid protein propagation is still a matter of intense debate in the literature. Contrary to previous work we were able to show, using high resolution microscopy methods developed in the group, how small quantities of healthy Tau deposited on the outside of brain cells get ingested by the cells, and that this process of ingestion causes the protein to misfold and aggregate into insoluble clumps. Crucially these aggregates then trigger the endogenous, 'healthy' Tau that is naturally present in neurons to misbehave and co-aggregate with the ingested Tau.
Unlike other studies in the field on this theme we have not used aggregates as a starting point for these propagation studies and we used endogenous protein levels and not over-expression systems, which can bias the outcome of such investigations. Not only were we able to observe, for the first time, how monomeric Tau is taken up by cells but also that the associated process of endocytosis, the uptake of proteins by lipid vesicles, is a likely event to trigger the first steps of Tau aggregation. This has potentially very significant consequences for the molecular pathology of Alzheimer's Disease: The mere process of endocytosis of Tau may be enough to trigger its 'misbehaviour'.
Tau is normally an intracellular protein and does no harm, however, if for some reason it is translocated to the outside of cells, our studies suggest that this could initiate the aggregation cycle that is linked to the disease. It also offers a potential explanation for the known fact that repeated head injury, e.g. sustained during contact sport, is connected to the onset of Tau related diseases: Neurons which die during head trauma release Tau into the extracellular space and from this point on Tau ingestion by adjacent, healthy neurons, could trigger nucleation and co-aggregation of endogenous Tau.
The findings were enabled by our development of a fluorescence lifetime sensor that reports on the aggregation state of amyloids in vivo. We have also made use of a novel two colour direct stochastic optical reconstruction microscopy (dSTORM) technique introduced by our group for amyloid research, a technique that is about to be published in our upcoming publication in Nano Letters.
This study featured on the University of Cambridge front website, the Alzheimer’s Research UK news page, and national / international press.
The work was funded through generous support by Alzheimer’s Research UK, the Medical Research Council and the Wellcome Trust.

Dr. Claire Michel, one of the investigators on the project, at work on the Fluorescence lifetime imaging microscope.
