Structural analysis of herpes simplex virus by optical super-resolution imaging. Laine R F. Albecka A, van de Linde S, Rees E J, Crump C M, Kaminski C F, Nature Communications 2015, 6, article number 5980.
DOI: 10.1038/ncomms6980 |
Abstract
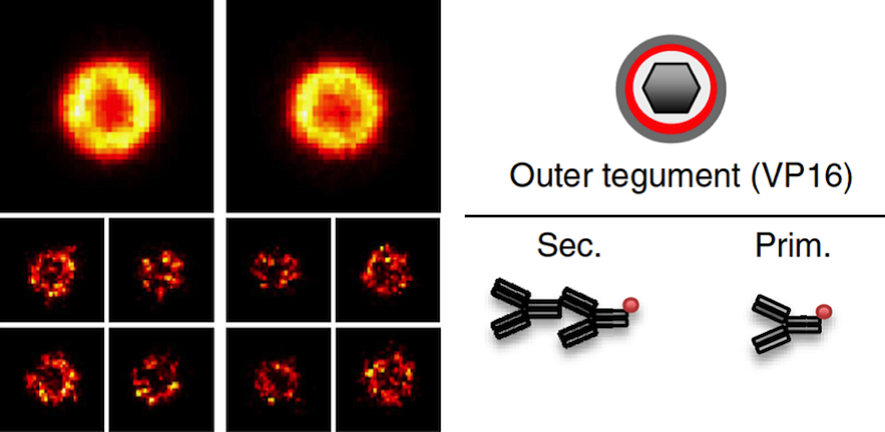
Herpes simplex virus type-1 (HSV-1) is one of the most widespread pathogens among humans. Although the structure of HSV-1 has been extensively investigated, the precise organization of tegument and envelope proteins remains elusive. Here we use super-resolution imaging by direct stochastic optical reconstruction microscopy (dSTORM) in combination with a model-based analysis of single-molecule localization data, to determine the position of protein layers within virus particles. We resolve different protein layers within individual HSV-1 particles using multi-colour dSTORM imaging and discriminate envelopeanchored
glycoproteins from tegument proteins, both in purified virions and in virions present in infected cells. Precise characterization of HSV-1 structure was achieved by particle averaging of purified viruses and model-based analysis of the radial distribution of the tegument proteins VP16, VP1/2 and pUL37, and envelope protein gD. From this data, we propose a model of the protein organization inside the tegument.
